-
In Same Day, UCB Lands Two FDA Drug Approvals in Autoimmune Diseases
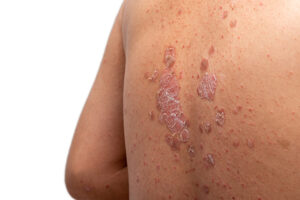
FDA approval of UCB’s Bimzelx gives the Belgian drugmaker a contender in the crowded plaque psoriasis therapies market. In the rare disease generalized myasthenia gravis, the agency approved Zilbrysq, a UCB drug with advantages over two blockbuster AstraZeneca drugs addressing the same target.
-
Thermo Fisher Adds Proteomics to Its Portfolio Through $3.1B Olink Acquisition
Thermo Fisher Scientific is acquiring Olink, a company that provides proteomics analysis tools and services used in drug discovery research. Olink’s growing revenue will help shore up a Thermo Fisher business segment whose sales have fallen due to declining Covid-19 testing demand.
-
Jorie Healthcare CEO Shares Why Automation is Critical to Revenue Cycle Management
The revenue cycle management business is using AI tools to automate cumbersome tasks to help hospitals operate more efficiently. It’s beginning to attract the attention of major healthcare organizations.
-
Roche Deal Aims to Get Molecular Glues to Stick to Elusive Cancer, Neuro Targets
Roche is paying $50 million up front to begin a drug R&D alliance with Monte Rosa Therapeutics, a biotech whose molecular glue technology could address targets previously deemed undruggable. It’s Roche’s second such deal in the past month.
-
Pfizer Shaves Revenue Projections, Starts Cost-Cutting Plan Amid Lower Covid-19 Product Demand
Pfizer lowered its revenue projections for 2023, a change it attributed to declining demand for its Covid-19 vaccine, Comirnaty, and antiviral drug, Paxlovid. Citing this revenue decline, Pfizer is implementing a companywide cost-cutting plan projected to save $3.5 billion.
-
Artificial Intelligence, BioPharma, Health Tech
FDA Forms New Digital Health Advisory Committee to Cover Growing Role of Tech
The FDA’s digital health advisory committee will discuss technologies such as artificial intelligence and machine learning, virtual reality, and digital therapeutics as well as topics like decentralized clinical trials and patient-generated health data. Committee member nominations are due in December.
-
Pfizer Expands in Immunology With FDA Approval of New Ulcerative Colitis Drug
Pfizer’s new FDA-approved ulcerative colitis drug Velsipity comes from its $6.7 billion Arena Pharmaceuticals acquisition. The small molecule will compete against blockbuster Bristol Myers Squibb drug Zeposia, which addresses the same target.
-
EU Orders Grail Sale; Illumina Continues Appeals But Plans for Multiple Outcomes
European Union regulators ordered Illumina to divest Grail. Illumina is appealing antitrust findings in Europe and the U.S., but the DNA sequencing giant revealed that divesting all or part of Grail is an option even if it wins both legal challenges.
-
BioNTech Turns to China Again for Cancer Drugs, Paying $70M to Partner on an ADC
BioNTech gains rights to a MediLink Therapeutics antibody drug conjugate that targets tumors expressing the HER3 protein. The deal comes six months after the German company entered the ADC field by acquiring rights to two therapeutic candidates from DualityBio.
-
Navigating Healthcare’s Data Revolution: Priorities, Opportunities, and Challenges for Health Systems
Arcadia recently partnered with HIMSS Market Insights to survey executives, IT, technology, and clinical leaders. Here’s what we found.
-
Pharma, Artificial Intelligence, BioPharma
Sanofi Adds Biologics to Its AI Ambitions, Striking Up R&D Alliance With BioMap
Formed by Baidu founder and CEO Robin Li, BioMap uses artificial intelligence technology to glean insight into proteins to guide biologic drug discovery. The startup is the latest company to join a growing list of Sanofi partners as the pharmaceutical giant continues investing in AI-enabled drug discovery.
-
Bayer’s New $250M Cell & Gene Therapy Site Signals More Investments to Come
Bayer opens its new cell therapy manufacturing facility as it prepares a Parkinson’s disease cell therapy for Phase 2 testing. The new 100,000 square foot facility also has space for manufacturing other cell therapies in the pharma giant’s pipeline.
-
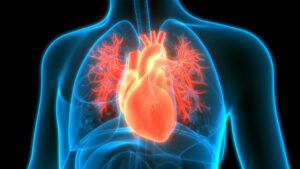
FDA Rejection Is a Delay, Not a Detour for Alnylam’s Aim to Treat Heart Disorder
Despite an affirmative FDA advisory committee vote, the agency declined to approve Alnylam Pharmaceuticals’ Onpattro for treating the heart complications caused by a rare, inherited protein disorder. But Alnylam has other drugs candidates for the disease, including one expected to post Phase 3 data in the first half of 2024.
-
Bristol Myers Squibb Bolsters Its Cancer Presence With $4.8B Mirati Acquisition
Acquiring Mirati Therapeutics brings Bristol Myers Squibb Krazati, one of two FDA-approved therapies addressing a KRAS cancer mutation. The deal comes as BMS looks to add revenue-generating products as patent expirations loom for several of its cancer products.
-
Takeda to Pull Lung Cancer Drug from Market After Failed Confirmatory Study
Takeda Pharmaceutical drug Exkivity failed the confirmatory study required of its 2021 accelerated approval. Our recap of other recent regulatory developments includes a partial clinical hold on a cancer drug, a Covid-19 vaccine authorization, and several drug approvals in the U.S. and beyond.